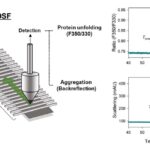
Principle of Differential Scanning Fluorimetry
Differential Scanning Fluorimetry (DSF) is a biophysical technique used to study the thermal stability of proteins by monitoring changes in fluorescence intensity. The principle of DSF is based on the fact that the fluorescence of a dye, such as SYPRO Orange, increases upon binding to hydrophobic regions of denatured proteins. This increased fluorescence signal can be used to detect protein unfolding during thermal denaturation.
DSF is performed using a specialized instrument, known as a differential scanning fluorimeter. This instrument measures the difference in fluorescence intensity between a dye-bound protein sample and a reference sample (typically buffer or water) as the temperature is gradually increased. As the protein undergoes thermal denaturation, the fluorescence intensity of the dye-bound protein sample increases, while the reference sample remains constant. The resulting data can be plotted as a melting curve, which represents the change in protein stability as a function of temperature.
learn more: circular dichroism

Nano differential scanning fluorimetry (nanoDSF) (Kim et al., 2022)
Benefits of Differential Scanning Fluorimetry
The utilization of differential scanning fluorimetry (DSF) is a paramount approach for assessing protein stability, possessing numerous benefits compared to alternative methods. Primarily, DSF necessitates meager amounts of protein, signifying that it is an impeccable technique for high-throughput screening applications. Secondly, it is a label-free method that does not require any modification of the protein, a factor that could potentially influence its stability, thereby rendering DSF a prime choice. Thirdly, DSF has an exceptional degree of sensitivity, capable of detecting subtle alterations in protein stability that may elude other approaches.
Moreover, DSF is a technique that can be characterized as being uncomplicated and expeditious when compared to other biophysical methods such as circular dichroism and NMR spectroscopy, which can be arduous and necessitate the usage of specialized equipment. As an added benefit, DSF can be employed to scrutinize a plethora of conditions, including pH, salt concentration, and ligand binding, in order to deduce their impact on protein stability.
DSF can also be employed to study the repercussions of mutations on protein stability. Mutations that affect the stability of a protein can have profound ramifications for its functionality and activity. DSF can be used to determine the influence of mutations on the thermal stability of a protein and can furnish valuable information for drug discovery and protein engineering.
Data Analysis for Differential Scanning Fluorimetry
The data obtained from a DSF experiment can be analyzed to determine important protein stability parameters, such as melting temperature (Tm), which is the temperature at which 50% of the protein is denatured. The shape of the melting curve can also provide information about the cooperativity of protein unfolding and the presence of multiple unfolding transitions.
Data analysis for DSF involves fitting the melting curve to a mathematical model to obtain the Tm and other parameters. Several software programs are available for data analysis, including commercial software packages like ProteOn Wizard and open-source software like NanoTemper Analysis.
Applications of Differential Scanning Fluorimetry
DSF has many applications in protein research and drug discovery. DSF can determine the stability of proteins under different conditions, providing valuable information for protein engineering and formulation development. It can also be used to screen for ligand binding, study protein-protein interactions, and determine stabilization conditions for difficult-to-handle proteins.
In drug discovery, DSF can be used to screen for ligand-protein binding, identify potential drug candidates, and determine the mechanism of action of a drug. It can also be used to develop biosimilars to demonstrate similarity to reference products and to optimize formulation conditions.

Protocol for high-throughput screening and validation of compoundsusing differential scanning fluorimetry (Støve et al., 2020)
References
- Kim, Soo Hyun, et al. “Nano differential scanning fluorimetry-based thermal stability screening and optimal buffer selection for immunoglobulin G.” Pharmaceuticals 15.1 (2022): 29.
- Støve, Svein I., et al. “Differential scanning fluorimetry in the screening and validation of pharmacological chaperones for soluble and membrane proteins.” Protein Homeostasis Diseases. Academic Press, 2020. 329-341.