IMARC Group, a leading market research company, has recently released a report titled “Bleeding disorder testing market Size, Share, Trends and Forecast by Product, Material, Distribution Channel, Pricing, End-User, and Region, 2025-2033.” The study provides a detailed analysis of the industry, including the bleeding disorder testing size, share, trends, and growth forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Market Overview
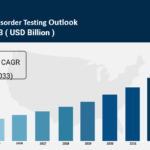
The global bleeding disorder testing market size was valued at USD 101.4 Million in 2024 and is expected to reach USD 184.2 Million by 2033, growing at a CAGR of 6.9% during the forecast period 2025-2033. The market growth is driven by the rising prevalence of bleeding disorders, an increasing number of traumas and surgeries, extensive research and development activities, and supportive government policies. The Bleeding Disorder Testing Market focuses on diagnostic processes for abnormal bleeding tendencies including assays, tests, and genetic evaluations.
Study Assumption Years
● Base Year: 2024
● Historical Year/Period: 2019-2024
● Forecast Year/Period: 2025-2033
Bleeding Disorder Testing Market Key Takeaways
● Current Market Size: USD 101.4 Million in 2024
● CAGR: 6.9% during 2025-2033
● Forecast Period: 2025-2033
● The rising prevalence of bleeding disorders globally is a significant market driver.
● Increasing trauma cases and surgeries require preoperative bleeding disorder testing.
● Extensive R&D initiatives including next-generation sequencing and POCT devices are fueling market growth.
● Supportive government policies enhance awareness, reimbursement, and access to high-quality treatments.
● North America dominates the market due to advanced healthcare infrastructure and high disease prevalence.
Claim Your Free “Footwear Market” Insights Sample PDF:https://www.imarcgroup.com/bleeding-disorder-testing-market/requestsample
Market Growth Factors
The growing prevalence of bleeding disorders worldwide serves as a major driver for the bleeding disorder testing market. Testing methods efficiently diagnose and determine the severity of conditions like hemophilia A and B, aid in treatment monitoring, and facilitate personalized therapeutic dosing. Additionally, testing supports accurate identification of Von Willebrand disease and platelet function disorders by measuring antigen levels, activity, and receptor function. These diagnostic capabilities enhance clinical management and patient outcomes, thereby propelling market demand.
The increasing number of trauma cases and surgical procedures globally is further accelerating market growth. Preoperative screening is essential to identify patients at risk of excessive bleeding during surgery, guiding perioperative management and hemostatic strategies. Bleeding disorder tests also enable safe blood product selection during transfusions and support anticoagulant therapy monitoring in trauma patients. The management of postoperative bleeding complications through testing also underscores its critical role in medical care.
Extensive research and development activities are fostering innovation in bleeding disorder testing. The introduction of next-generation sequencing permits simultaneous gene analysis for precise diagnosis and personalized treatment planning. Moreover, point-of-care testing devices that offer quick, on-site results improve patient convenience and immediate clinical decision-making. The integration of artificial intelligence to analyze large datasets and detect patterns, along with digital microfluidics technology enhancing assay efficiency, is driving the advancement and adoption of bleeding disorder diagnostic tools.
Market Segmentation
By Product Type:
● Reagents and Consumables: Represent the largest segment due to their role in sample preparation and assay accuracy. Increased demand for repeated testing and customized reagents tailored to specific methodologies drives growth.
● Instruments: Not explicitly detailed in report but form the other core product type in diagnostics.
By Indication:
● Hemophilia A: Dominates the market as the most common and severe bleeding disorder requiring comprehensive diagnostic approaches including factor assays and genetic testing.
● Hemophilia B: Not detailed but included as a key indication.
● Von Willebrand Disease: Testing includes measurement of antigen levels and factor VIII.
● Idiopathic Thrombocytopenic Purpura: Part of the indication segments.
● Others: Include rare bleeding disorders involving specific clotting factor deficiencies.
By End User:
● Hospitals and Clinics: Largest segment due to presence of specialized medical professionals and advanced diagnostic facilities enabling comprehensive testing and quick results.
● Diagnostic Centers: Included as primary end users.
● Others: Not further specified.
By Region:
● North America: Largest market segment attributed to advanced healthcare infrastructure, high prevalence of bleeding disorders, and supportive policies.
● Europe, Asia Pacific, Latin America, Middle East and Africa: Included as regional markets with specific countries listed.
Regional Insights
North America leads the bleeding disorder testing market, accounting for the largest share globally. This dominance is due to the region’s advanced healthcare infrastructure comprising hospitals, clinics, and diagnostic facilities equipped with state-of-the-art technologies. The high prevalence of bleeding disorders like hemophilia and von Willebrand disease, combined with supportive government policies and rising consumer awareness towards early diagnosis, significantly fuels market growth in this region.
Recent Developments & News
● In March 2023, Abbott Laboratories received US FDA clearance for a blood test detecting traumatic brain injuries.
● In May 2022, HORIBA Ltd unveiled a new generation of hemostasis analyzers and ready-to-use reagents at ISTH 2022.
● In January 2023, Precision BioLogic Incorporated announced FDA clearance and U.S. launch of new hemophilia-related diagnostic products.
Key Players
● Abbott Laboratories
● DiaPharma Group Inc.
● HORIBA Ltd
● HYPHEN BioMed (Sysmex Corporation)
● Precision BioLogic Incorporated
● PreventionGenetics (Exact Sciences Corporation)
● Roche Holding AG
● Siemens Healthineers AG (Siemens AG)
● Thermo Fisher Scientific Inc.
● Werfen S.A.
Ask an Analyst : https://www.imarcgroup.com/request?type=report&id=9003&flag=C
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302