Real-World Evidence Market size was valued at USD 1.81 Bn in 2024, and the total Real-World Evidence Market revenue is expected to grow at a CAGR of 14.3 % from 2025 to 2032, reaching nearly USD 5.27 Bn.
Market Estimation & Definition
The Real-World Evidence (RWE) Market is on a firm upward trajectory. One forecast estimates the RWE solutions market will expand from approximately USD 5.4 billion in 2025 to USD 10.8 billion by 2030, achieving a compound annual growth rate (CAGR) of around 14.8%. Another projection suggests growth from USD 20 billion in 2025 to USD 48 billion by 2032, at a CAGR of 13.3%. A third perspective pegs the base market at USD 3.3 billion in 2024, with a rise to USD 10 billion by 2035, reflecting a CAGR of approximately 10.6%.
RWE refers to clinical insights derived from analyzing real-world data—such as electronic health records, insurance claims, registries, patient-reported outcomes, and wearable device metrics. This evidence supplements traditional clinical trials, enabling more holistic decision-making in clinical, regulatory, and commercial contexts.
Secure your sample copy of this report immediately! https://www.stellarmr.com/report/req_sample/real-world-evidence-market/2675
Market Growth Drivers & Opportunity
-
Shift Toward Value-Based Healthcare
The industry’s shift away from volume-based models toward value-based outcomes is pressing payers and providers to use RWE for pricing, reimbursement, and performance assessments. -
Digital Health Data Proliferation
Widespread adoption of electronic health records, insurance databases, registries, and wearable devices is generating rich, actionable data for analytics and RWE. -
AI and Advanced Analytics Integration
Adoption of AI, machine learning, and data modeling tools has enhanced the ability to extract insights from complex real-world datasets, including using synthetic cohorts and external control arms to streamline drug development. -
Regulatory Endorsement and Emerging Guidelines
Regulatory agencies are increasingly recognizing the role of RWE in approvals and safety monitoring. Frameworks and guidance for using real-world data in medical decision-making and post-market surveillance are facilitating broader adoption. -
Growth in Emerging Markets
Regions such as Asia-Pacific and Latin America are showing rapid uptake due to expanding healthcare infrastructure, rising healthcare R&D, and untapped data repositories enabling local studies.
Segmentation Analysis
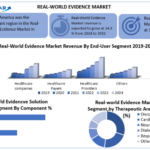
By Component
-
Services: Accounted for the largest share in 2024 (~57%), including analytics, consulting, study design, and clinical protocols.
-
Data Sets: Encompass claims, clinical, pharmacy, patient-reported, and wearable data—expected to grow the fastest during the forecast period.
By Application
-
Drug and Device Development & Approvals: Leading use case, as companies integrate RWE into regulatory submissions and trial designs.
-
Reimbursement and Regulatory Decision-Making: Fastest-growing segment, used increasingly in coverage decisions and policy frameworks.
-
Safety Monitoring (Post-Market Surveillance): Valuable for ongoing monitoring of adverse events and pharmacovigilance.
-
Medical Device Evaluations: Growing RWE use in device clearance and performance monitoring.
By End User
-
Pharmaceutical & Medical Device Companies: Largest users for trial optimization and regulatory interactions.
-
Healthcare Payers: Increasingly deploying RWE to assess outcomes and negotiate value-based contracts.
-
Healthcare Providers: Leveraging insights to guide patient care and outcomes tracking.
By Region
-
North America: Largest market, benefiting from favorable regulations and high R&D intensity.
-
Asia-Pacific: Strongest growth prospects, fueled by digital transformation and government support.
-
Europe, Latin America, MEA: Emerging opportunities driven by data infrastructure development and regulatory interest.
To further investigate this topic, please navigate to the following link: https://www.stellarmr.com/report/real-world-evidence-market/2675
Country-Level Analysis
United States
As the dominant regional market, the U.S. is driven by high pharmaceutical R&D spend, mature data ecosystems, and early regulatory frameworks supporting RWE integration.
European Markets (e.g., Germany, UK)
Well-positioned with structured healthcare systems, regional RWE initiatives, and regulatory strategies that leverage data for public health and policy decision-making.
Asia-Pacific (e.g., China, India)
Rapidly evolving, with expanding clinical trial activity, increased RWE adoption, and growing interest in value-based care frameworks.
Competitive Analysis
The RWE market features both specialized service providers and end-to-end platforms. Leading organizations include global players offering data access, analytics, consulting, and study execution services.
Strategic Focus Areas:
-
AI, Modeling, and Synthetic Controls: Companies leveraging advanced analytics are enabling faster, more robust insights.
-
Interoperability and Federated Data Handling: Vendors capable of harmonizing diverse datasets and complying with privacy regulations have an edge.
-
Collaborations and Partnerships: Alliances among CROs, healthcare systems, and analytics firms help build integrated evidence ecosystems.
-
Subscription & Value-Based Pricing Models: Engagements increasingly structured around ongoing analytics and outcome performance.
View Popular Topics Now :
Germany Ivf Services Market https://www.stellarmr.com/report/Germany-IVF-Services-Market/1587
Mexico Ivf Services Market https://www.stellarmr.com/report/Mexico-IVF-Services-Market/1589
Conclusion
Projected to grow from USD 5–20 billion in the mid-2020s to potentially USD 11–48 billion by the early 2030s, the Real-World Evidence Market demonstrates robust CAGR trajectories ranging from 10% to 15%, depending on horizon and basis.
Key Takeaways for Stakeholders:
-
Life Science Companies: Can accelerate development timelines and improve regulatory positioning via RWE-supported submissions.
-
Payers & Providers: Can better manage value-based payments and outcomes tracking through integrated RWE insights.
-
Policymakers & Regulators: Should continue to define clear frameworks and standards to streamline RWE utilization.
-
Investors: The space offers attractive growth opportunity, especially in scalable platforms and emerging regional markets.
As healthcare evolves toward data-driven, patient-centric models, RWE stands as a transformative force enabling smarter, more efficient decision-making across the care continuum.
About Stellar Market Research:
Stellar Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include science and engineering, electronic components, industrial equipment, technology, and communication, cars, and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Contact Stellar Market Research:
S.no.8, h.no. 4-8 Pl.7/4, Kothrud,
Pinnac Memories Fl. No. 3, Kothrud, Pune,
Pune, Maharashtra, 411029
+91 20 6630 3320, +91 9607365656